On February 1, 2013, Hiroshima University established two new programs: the “Distinguished Professors” (DP) program and the “Distinguished Researchers” (DR) program. Individuals who are part of these programs are recognized as senior and junior faculty members respectively, who are engaged in extraordinarily distinguished research activities.
A Conversation with Distinguished Researcher Kiyofumi Katagiri

Can you tell me your name and specialist subject?
My name is Kiyofumi Katagiri. My major is materials chemistry focusing on hybrid materials and nanomaterials.
What are you working on right now?
One of my characteristic is structural color. Usually we think of color as formed from dyes or pigments, which absorb certain light wavelengths, but structural color is different – it is caused by molecules scattering light at different wavelengths. For example, opal stones are very colorful when held up to the light and this color results from the opal's nanoscale structure. We prepare such colors artificially.
How do you create these structural colors?
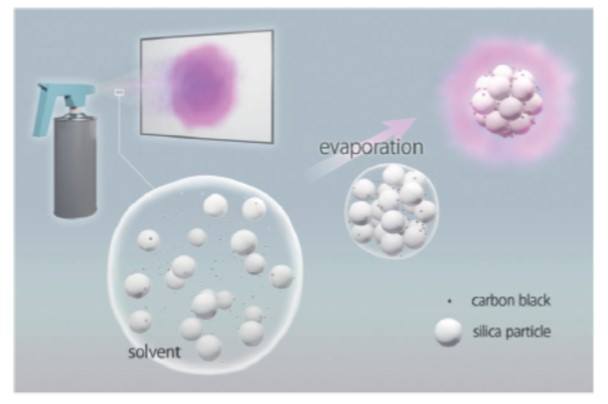
We use silica particles with a sub micro diameter. When these silica particles are structurally assembled, color is primarily due to light being scattered at varying wavelengths – just as in an opal stone. We can control which colors appear by varying the size of the silica. Particles involved.

What are the benefits of structural colors over traditional dye based colors?
If you consider an old poster, usually the color will fade over time because the pigments will decompose with exposure to UV light. This is not the case in an opal which derives it's colorful appearance from the dispersion of light by the crystalline assembly of silica particles. – It's color never diminishes.
If you look at an opal from different angles, the color appears to change – this aesthetic is the reason they are sought after.Each particle irradiates color, but this depends on the angle it is viewed from. Looking one way it is blue, another it is green.
This property is sometimes suitable for applications such as automobile paint or perfume bottles where varying color works aesthetically.However, for other uses we need color to remain constant and stable.
How is such color stability achieved?
We take our cue from nature! The natural world is made up of structural colors. Take a blue bird for example; its feathers are not dye based and yet it always appears blue, whatever angle it is viewed from. The blue feathers use structural color – but its structure is not crystalline and it only reflect short range light waves, as opposed to the long range light reflected from a crystalline structure.
However, when we try to replicate such a structure using silica particles, the resultant color appears extremely faint. The color almost appears white due to the very strong light scattering properties. This leads to an incoherent hue.



So what's the secret to the bird's vibrant color?
Well the bird has a layer of black melanin under its color structure and this absorbs much of the scattered light. The scattered light is suppressed and a vibrant blue is detected. To mimic this phenomenon we include black carbon into our structural particles.
The more carbon added, the stronger the resultant color.
What in your career are you most proud of?
One project that stands out is my work on liposomes. Liposomes are spherical vesicles made up of lipids that we can use to deliver drugs inside the body. They are effectively organic capsules that protect the drugs as they pass through the body, but they are not controllable, reducing the effectiveness of the drugs. I developed a new photoactive type – using thermosensitive titanium oxide – that decomposes when exposed to UV radiation, and so releases drugs on cue.
The problem is that UV light cannot penetrate the body and it also damages bodily tissues! We have worked to resolve this by working on the nanostructure of the vesicles involved so that their permeability changes in response to increased magnetic field. In this case we can use MRI which is safe. The increased magnetic field increases the energy in the vesicle molecules to an extent that the outer envelope breaks down. The drugs can then be released when and where required.
Overall, why do you think your research is important for society?
We strive to come up with new and better materials. While the materials we have available to us currently provide us with a comfortable lifestyle – they still cause many problems to the environment. Our aim is always to improve things for the next generation. One major project we are working on is the development of artificial photosynthesis to provide green energy in solar cells. Another is a photocatalyst to assist in splitting water into its oxygen and hydrogen components. H2 will be an important energy source in the future.
Have you ever experienced any disappointments in your career?
Chemical research is full of setbacks as we rely on trial and error – we are never completely unsuccessful, but we are somewhat unsuccessful every day! It is how we reach our target. It is kind of like climbing a mountain, we know where the target is but there are many routes to get there, no one knows the correct route – and no route is wrong.
Can you tell me about your background?
I was born in Gifu prefecture, but after elementary school, I spent 12 years in Shimane prefecture. I returned to Gifu during my second year of high school. Later I entered Osaka Prefecture University where I stayed for four years in the Department of Applied Materials Science. I chose to work in the inorganic materials lab and worked on materials chemistry, including projects based on silica.
For graduate school I transferred to Nara Institute of Science and Technology where I was their first student of Graduate School of Materials Science. I started then working on organic lipid projects and then I fused my combined knowledge of both organic and inorganic chemistry to focus on hybrid material research for my PhD.
I did my postdoc fellowship at the University of Melbourne in Australia where I learned to assemble polymers. I returned to Japan in 2005 and spent one year in Toyohashi University of Technology as a postdoc fellow and in 2006 I got an assistant professor position in Nagoya University. I came to HU in 2011.
Did you like living in Melbourne?
It's very beautiful. There are many parks and the food offering is very diverse – Asian, Greek, Italian, Middle Eastern – a great place to eat out!
Do you recommend students and researchers spend time in other country?
Yes, but I think they should go as early as they can. In Japan researchers tend to go abroad after they have gained a position – in their 30s. We should go earlier as it becomes more difficult to master an overseas language, as we get older. But the academic environment in Japan makes it difficult to spend time abroad. If we do go, we then have to work harder and get many results and publications out if we are to regain a place on returning to Japan. Of course, it is much better if we can get a position abroad but few Japanese researchers can obtain tenure overseas. One of the reasons Japanese universities are falling in world rankings is due to a lack of international collaboration – We need to go abroad and make contacts and friends so that international collaboration is possible.
What is your work life like?
I come to the office every day before nine. After six I go home, have dinner with my child, and get her washed. I then return to the lab at 8 or 9pm and work until 12. I have many tasks to complete, not only research.
What do you like most about your work?
I like it all – the education and the research, also I am involved in sourcing funding for the university.
What do you like most about your work?
I like it all – the education and the research, also I am involved in sourcing funding for the university.
When you are not at work, what do you do? Do you have hobbies?
I like mountain trekking.I have little time to do it now but as a student I was part of the climbing society and I have trekked in Nepal.What I liked most about climbing was how I could cut myself off from the world by climbing the No one could reach me there and I left my stresses in the world down below.But this is not the case now – we can even get mobile signal in the high peaks!
Were you interested in science when you were young?
Yes. My father worked in academia, so I knew it was a good career option, and my brother is also a professor – of pharmacy. I guess it runs in the family.
What's best thing about working at HU?
As faculty we get to work directly with many students and they study very hard. Of course, we are in such a remote location here so there is little to distract them!
Are there any downsides?
Not so many – just the lack of local competition perhaps discourages the students and staff to really push themselves. I think we have a great potential to do even better here but we need to push ourselves harder.
With that in mind, HU recently set the aim of becoming one of the top 100 manufactures in the world – is this a realistic prospect?
Firstly, we will have to change our mindset – it will be very challenging, but it is not impossible!
Finally, what advice do you have to students joining HU?
Don't set yourself a limit – everything can be challenged!


Read more articles on Distinguished Professors / Researchers here
Written by Richard J. O'Connor (Hiroshima University Science Communication Fellow)
Find out more about Associate Professor Katagiri, and how to contact him, here: http://seeds.office.hiroshima-u.ac.jp/profile/en.9998aa139fc87e1f520e17560c007669.html

 Home
Home