On February 1, 2013, Hiroshima University established two new programs: the “Distinguished Professors” (DP) program and the “Distinguished Researchers” (DR) program. Individuals who are part of these programs are recognized as senior and junior faculty members respectively, who are engaged in extraordinarily distinguished research activities.
Distinguished Professor Yohsuke Yamamoto is efficient in his movements around the Chemistry Department of Hiroshima University. The hallway is dimly lit between his office stuffed with books and the seminar room used as a break room by students. He prefers to speak in the seminar room rather than his office, but he points out that the so-called seminar room is too small to hold everyone at the weekly lab seminars he organizes for his students. Those happen in a large lecture room at the opposite end of the hall.
Yamamoto had a strong interest in organic chemistry in high school, so he earned Bachelor’s Master’s and Ph.D. degrees in organic chemistry at the University of Tokyo between 1973 and 1982, and then immediately started his professional career as an organic chemist at Hiroshima University. In 1989 he spent a year at Vanderbilt University (Tennessee, USA) with Professor James Cullen Martin, a revered physical organic chemist. He has led the Organic Main Group Element Chemistry research group of the Department of Chemistry in the Graduate School of Science at Hiroshima University since 2001.
A CARBON WITH FIVE BOND
“The chemistry I do isn’t a common study area for other research groups, so there is less competition, meaning there is less time pressure. I do not have to always urge the students, ‘We must do this as soon as possible.’ We have the flexibility to go in many directions with our research and be free to do what we find interesting,” says Yamamoto.
The central focus of Yamamoto’s research is to find ways of stabilizing very reactive organic species. Unlike species of living things identified by biologists, a chemical species refers to the same element in its different forms. Table salt, NaCl, is a molecular species, but when dissolved in water, the atoms dissociate and become the charged ionic species Na+ and Cl-. Very reactive species will readily undergo chemical reactions with other species if they are around and unstable species will degrade quickly after they form. The premise of Yamamoto’s research is to design chemical environments to stabilize supposedly highly reactive or unstable species so they can be isolated and studied in laboratories.
Usually, stabilizing reactive or unstable species involves building a framework with clever chemical environments that stabilize the species. The reactive species Yamamoto has stabilized during his career have challenged the fundamental chemistry dogma dictating what type of bonds an element can form. The work is time-consuming and complex, which Yamamoto believes contributes to its lack of popularity among other researchers.

Yamamoto speaking about the penta-coordinated carbon, which he worked on in 1999.
“One of the biggest accomplishments for me was making a stable penta-coordinated carbon in 1999 while I was an associate professor working under Professor Kin-ya Akiba. We made carbon stable with five bonds by building a chemical backbone, a framework, that puts carbon in a unique situation where having five bonds is actually more stable than only four bonds.”
“One of the biggest accomplishments for me was making a stable penta-coordinated carbon in 1999 while I was an associate professor working under Professor Kin-ya Akiba.We made carbon stable with five bonds by building a chemical backbone, a framework, that puts carbon in a unique situation where having five bonds is actually more stable than only four bonds. ”
When an element possesses more bonds than is expected by the octet rule, it is labeled as hypervalent. The chemical architecture of the framework Yamamoto built to stabilize the penta-coordinated carbon can be fine-tuned to stabilize other elements, such as boron, in a hypervalent state.

One of the air-tight chemical hoods in Yamamoto’s laboratory. Equipment like this is needed to work with chemicals affected by exposure to oxygen, like the anti-aromatic porphyrin ring.
DOING WHAT IS INTERESTING
Yamamoto’s research interests have expanded beyond the so-called “main group” elements, which include organic elements like boron, carbon, nitrogen, phosphorus, antimony, sulfur, and selenium, located in Groups 13 to 16, the columns on the right-most third of the periodic table. Now, his work also includes transition metals from the middle columns of the periodic table, like nickel, iridium, rhodium and palladium.
“I started on very narrow research about the main group elements’ chemistry as an undergraduate and graduate student. But then, over time, my interests broadened. Many times I was asked, ‘Why are you doing such kind of things?’ And my answer is, "Because I find it interesting."
What interests Yamamoto is the possibility that the chemical frameworks he created could be applied in ways beyond the original intent of his research. The puzzle of figuring out how to adapt the backbone to new applications provides a worthwhile challenge.
“But now I have a very big problem. I must find a way to summarize my research before I have to retire. Many students now are asking, ‘You have so many fields of research. How will you summarize your work at your retirement?’ But so far I tell them, ‘It’s OK. I don’t mind.’ I still have time.”
A CARBON WITH TWO BONDS?
One project that he knows he might not have enough time to solve is a decade-long research puzzle that continues to go unfinished.
“This project is very, very difficult. But we are starting to have some success, after 10 years of trying,” says Yamamoto.
In addition to the traditional four bonds and hypervalent five bonds, carbon can also sometimes make only two bonds by forming a structure called a carbene. Carbenes are predicted to have two distinct electronic states called singlet and triplet. The singlet and triplet carbenes are expected to have distinct chemical properties.
Ernst Otto Fischer and Geoffrey Wilkinson received the Nobel Prize in Chemistry in 1973 for their research related to carbenes, and Yamamoto mentions speculation within the chemistry community that other researchers may soon receive the Nobel Prize for related follow-up accomplishments of singlet carbenes. However, currently triplet carbenes can exist only in solution because they are so unstable. Yamamoto’s goal is to create a way to stabilize a triplet carbene and isolate it as a crystalline solid or powder.
“In the early 2000s, a different Japanese research group set a world-record for observing a triplet carbene in solution for two weeks. If we can stabilize a triplet carbene in the solid-state, it will be like a gold medal,” says Yamamoto.
A STUDENT NETWORK AND CHEMICAL RINGS
Yamamoto’s research lab has always been staffed almost entirely by students. One of his former master students now teaches second and third-year high school chemistry and invites Yamamoto to speak to his students every year.

Yamamoto speaking about the importance of his student collaborators in the seminar room at Hiroshima University.
“Speaking to these very young students is my way of contributing to society. One of the high school students I spoke to probably four years ago is now a chemistry Master's student in another lab of the Department of Chemistry.”
Yamamoto has also hosted undergraduate summer students from the University of Alabama (Alabama, USA) as part of a personnel exchange program and has held an ongoing appointment as an Adjunct Professor there since 2013.
About 60 of Yamamoto's former Hiroshima University students joined his 60th birthday party, filling the local restaurant where they celebrated. A photo of a recent lab group excursion to the top of Mount Misen, located on the nearby island of Miyajima, is the desktop image on the computer behind Yamamoto in the seminar room.
“Sometimes when the results are not promising for a long time, students want to change focus and do something that can help them succeeded with their studies. They are frustrated, so it is important for me to consult with them about the direction of the research that I share with them.”
Despite the frustrations, sometimes great results can happen unexpectedly.
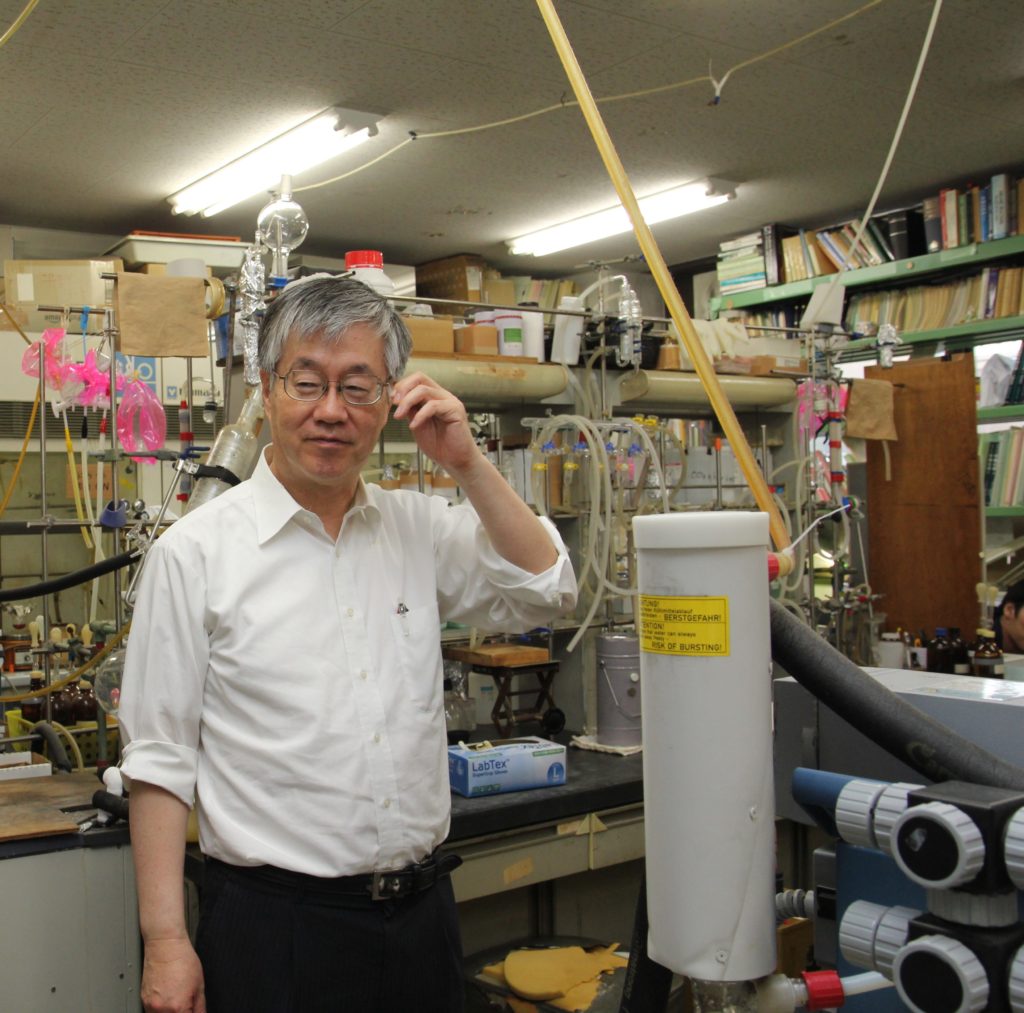
Yamamoto in one of the laboratories his group members use, where text books, glass ware, and baseball mitts compete for space. “The interdepartmental baseball game is next weekend, so the students are practicing when they have time. I’m on the reserves, usually just a commentator,” Yamamoto says.
Porphyrin rings are a structural group familiar to chemists and common in living organisms as a carrier of iron in blood. These rings are usually highly stable. A range of different elements can be supported in the center of the ring. However, no one had ever isolated a porphyrin compound with sulfur at the center. For no reason other than curiosity, Yamamoto decided to pursue the project with one of his Master’s degree students.
Porphyrin rings’ stability comes from the double bonds between the elements building the ring structure. The rings are held together with 18p aromatic bonds, where interaction among double bonds creates a stable network.
A theoretically predicted alternative arrangement for porphyrin rings relies on 16p anti-aromatic bonds, where interaction among double bonds creates an unstable network. But Yamamoto’s student was doing the research in winter, when it can regularly get quite cold in the Chemistry building.
“My student brought me this crystal, asking me to analyze it so he could have some good result before he graduated. Judging from the color of the crystal, I expected nothing interesting, so I did not want to do it. But, he was desperate so I obliged him.”
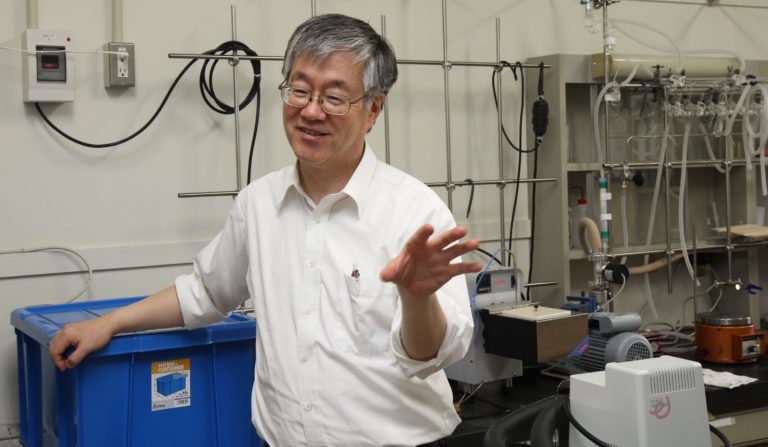
Yamamoto speaking about unexpected scientific discoveries.
After all the liquid used in the reaction had evaporated away, the crystal of a 16p anti-aromatic porphyrin ring remained. The cold temperature had allowed the unstable compound to resist degradation. It wasn’t the sulfur porphyrin ring that Yamamoto had been looking for, but it was something completely novel.
“Until we synthesized this 16p anti-aromatic porphyrin ring, it was only predicted to exist. So we verified the theoretical chemistry with our work and published several papers about it. But there is no practical application for such things. Not yet.”
ADAPTING TO APPLICATIONS
“I do pure research. I have not been interested in the applications of my research at all,” he says, with intense emphasis on the last two words. “That was how I was trained, to think I should focus only on the fundamental chemistry, that applications are a side business. But more recently, we have research funding to find functional molecules that can be applied to different problems of the world. There is a change in attitude among researchers. So, now I am actually looking for applications because they can be interesting too,” says Yamamoto.

Yamamoto considering the balance between fundamental research and practical applications.
Currently he is the Project Leader and Coordinator for a 5 years funding grant worth 111.8 million Japanese Yen (approximately 1 million US Dollars) from the Japanese government to search for potential functional applications of different kinds of synthesized organic molecules based on collaborative research among 73 different professors across Japan.
One successful functional application that Yamamoto’s research group published in early 2016 involves making a sulfur-radical based rechargeable battery without using metal. Such “eco-batteries” avoid the problems of relying on high-cost, limited supply metals, are able to re-charge much faster than current batteries because of how they store energy, and can continue to provide a reliable source of power in extreme cold.
The shift in Yamamoto’s outlook on the balance between fundamental research and research applications reflects a scientific cultural shift towards wanting research to be immediately useful for the public rather than remaining a purely philosophical pursuit for its own sake. There is a push to find applications of the fundamental knowledge of the elements that was so hard-earned through the passion and persistence of generations of chemists.
“We built a chemical backbone to stabilize very reactive species of sulfur or selenium. Then we realized that we could use these to make rechargeable batteries with no metal based on discussions among professors in the project. This was good, because it involves both new, fundamental chemistry and a functional application. I found the application is also very interesting especially with use of our originally designed compounds.”
Yamamoto says that his main motivation continues to be fundamental knowledge of the elements. Perhaps because fundamental knowledge has a longer legacy.
“If we can isolate a molecule and discover some new chemistry, that can be a very nice result. We don’t have so many chances to do it. If I have an opportunity to change what is written in the sorts of text books I read when I first studied organic chemistry as a high school student, then I will take it.”
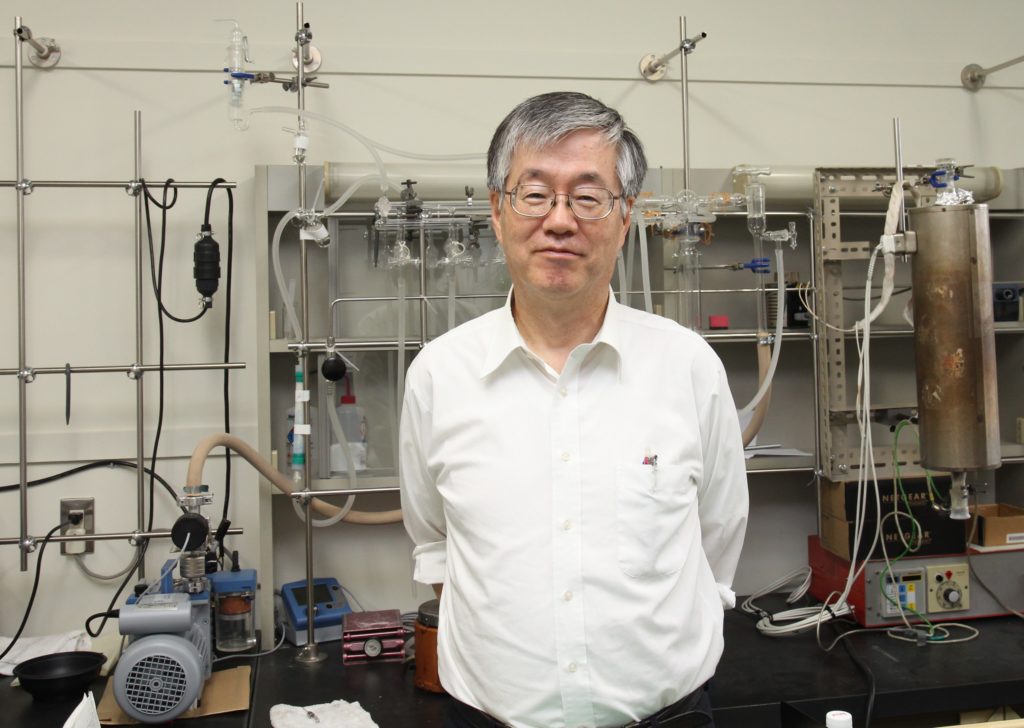
Professor Yohsuke Yamamoto of Hiroshima University in the lab of his faculty collaborator, Assistant Professor Rong Shang.

 Home
Home