On February 1, 2013, Hiroshima University established two new programs: the “Distinguished Professors” (DP) program and the “Distinguished Researchers” (DR) program. Individuals who are part of these programs are recognized as senior and junior faculty members respectively, who are engaged in extraordinarily distinguished research activities.
Interview of Distinguished Professor Takeharu Haino
The development of brand-new functional materials using reversible features of various supramolecules directed by "weak" non-covalent chemical bonds
Our focus on discovering various uncommon features associated with non-covalent chemical bonds in the development of unique functional materials
The focus of my research is supramolecular chemistry, which is a part of organic chemistry. General chemistry deals with molecular structures that are assemblies of atoms connected by chemical bonds (covalent bonds) where atoms share electrons. In contrast, supramolecular chemistry deals with supramolecules that are assemblies of molecules as a structural element which are weakly associated with each other by noncovalent bonds. The strong covalent bonds between atoms in molecules do not permit atoms to be interchangeable. In contrast, a unique characteristic of supramolecules is that these molecules can be replaced with copies of themselves.
Supramolecules exist all around us in various forms. Ice, for example, is a supramolecular structure. Water molecules are held together by hydrogen bonds, which are typical noncovalent interactions. When water molecules in ice are warmed, hydrogen bonds are weakened, which transforms ice into water. When water is warmed further, the hydrogen bonds are completely broken, then water molecules exist as a gas. The liquid crystals used in our TV screens are the same—controlling the way liquid crystalline molecules are assembled changes the colors and brightness of the screen. In this way, when liquid crystalline molecules come together to form supramolecules, they begin to display various properties.
I became interested in the boundaries between polymers and supramolecules, and have focused on research subjects to create brand-new polymer materials using supramolecular concepts that possess various functions. One representative example is a material with a self-healing function. Noncovalent bonds in supramolecules are weak and these bonds are reversible. When a healable supramolecular material can be cut and rejoined, the material interface can be restored by restoring temporarily broken bonds. One example of a product that already uses these damage-resistant properties is a smartphone case. My team and I went further, however, and developed a polymer material made from supramolecules that can return to its original state even when completely separated.
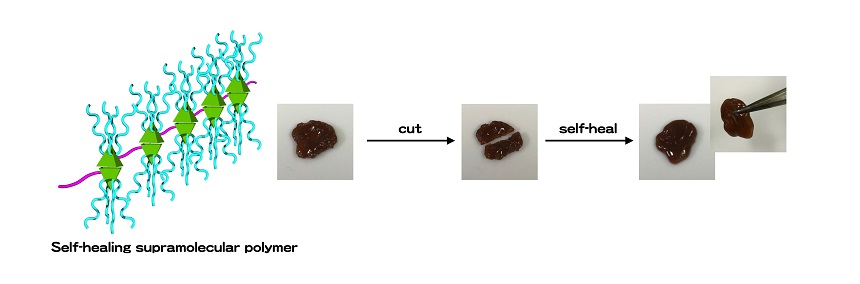
Prof. Haino and his team developed a polymer material with a self-healing function that can return to its original state even when completely separated
One other project we have worked on is the development of a supramolecular fullerene polymer. Fullerene is a highly stable spherical molecule made of sixty carbon atoms connected in a structure that resembles a football. We have successfully developed a molecule that grasps fullerene molecules similar to a baseball glove grasping a ball through noncovalent bonding. Using this molecule has enabled supramolecular assemblies of fullerene to be controlled at a size of just one nanometer—they can be freely arranged in a straight line or a level plane. Forming a three-dimensional structure using fullerene that has been arranged in a straight line can lead to the development of new supramolecular polymer materials.
Our strength is not our ability to combine existing materials—we possess the technologies to create new supramolecular polymers by designing molecules from scratch. It is like being able to develop order-made metal molds just one nanometer in size.
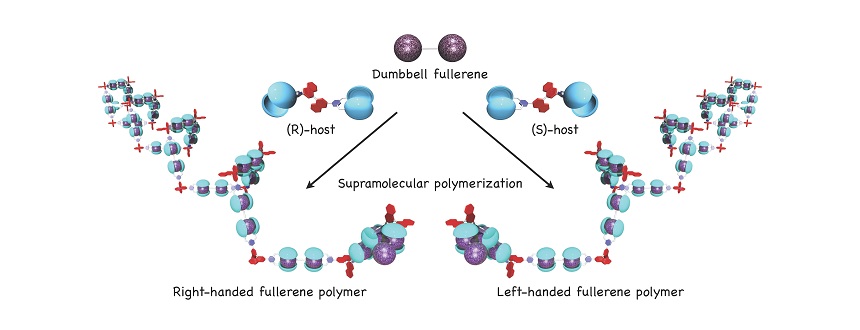
Helically organized supramolecular fullerene polymer formed by supramolecular association of dumbbell-shaped fullerene and twisted hosts
Our primary challenge is to uncover unknown properties of supramolecules
There is no material that has properties that perfectly satisfies our desires; this is why research work for the development of functional materials is so exciting. For example, the first cars were made mainly of wood and metal. These heavy materials have since been replaced with plastic, and with the progress of polymer science, this plastic continues to be replaced with lightweight, durable polymer materials. The lightweight properties achieved by polymer science continue to make huge contributions toward carbon neutrality. This isn’t to say that metals are inferior to polymers. Metals are heat resistant but prone to corrosion, while polymers are corrosion resistant—their innate properties are different. Rather than simply replacing one for the other, the applications for their use can be increased by maximizing their individual properties. My team and I thus set out to discover what properties we could make through developing polymer materials by making use of supramolecular polymers.
The foremost feature of supramolecular polymers is the above-mentioned self-heal functionality. We didn’t set out to create a material that could heal itself; however, the material that we created through noncovalent bonding just happened to have this capability. Ideally, we would like to entrust the relevant corporation with examining how to apply this functionality to society. As a researcher, I am more involved in discovering unexpected properties that are entirely different from those found in metals, wood, and polymers. For example, we might discover properties that are outside of common knowledge, such as materials that can heal themselves when exposed to light or materials that harden when warmed. It is discoveries like these that bring real excitement to a researcher.
A burning curiosity to solve chemical mysteries
We are also looking at the development of materials that can contribute to a low-carbon society, such as light-modulating materials that can block heat when applied to vehicle windows. At the very root of my role as a researcher is a curiosity to uncover the unknown. Supramolecules are formed through the assembly of molecules directed by intermolecular attractions. If we were to probe further, we could also say that viruses and even people, composed of an infinite number of molecules, are also supramolecules. However, it is still not known how these molecules come together to form living things. It is a field of research where the more you find out, the more interesting it becomes.
Elsewhere, I think it’s disappointing that chemistry is often seen as a branch of natural science that is particularly difficult to approach. For example, nuclear fusion, which generates sunlight, can be understood using chemistry, while life is a series of chemical reactions. Chemistry has just as many mysteries as the universe, and what is more, it is one of the most familiar areas of science to us. I hope we can attract more people to the field of chemistry and the excitement it holds.

Prof. Haino enthusiastically instructing students in the laboratory.

 Home
Home