On February 1, 2013, Hiroshima University established two new programs: the “Distinguished Professors” (DP) program and the “Distinguished Researchers” (DR) program. Individuals who are part of these programs are recognized as senior and junior faculty members respectively, who are engaged in extraordinarily distinguished research activities.
Interview of Distinguished Professor Hideshi Kawakami

Working to pinpoint causative genes and pathogenic mechanisms of the intractable disease ALS to open paths to developing treatments
Researching "the most difficult disease"
I am conducting genetic research to find causes and treatments for amyotrophic lateral sclerosis (ALS) and other intractable diseases such as Parkinson's disease, Alzheimer's disease, and spinocerebellar degeneration, which cause degeneration in neurons of the brain and spinal cord. ALS is a dreadful disease that gradually paralyzes all the muscles in the body, causing the affected person to become immobile and eventually die when they are no longer even able to breathe on their own. Several causative factors of Parkinson's disease and Alzheimer's disease were identified about 30 years ago, but the causes of ALS were largely unknown for a long time. When I was choosing a specialization, I thought about what kind of research I should do, and decided to focus on ALS because it is the least understood disease and still has no established treatment.
Currently, it is estimated that about 10% of ALS patients develop the disease due to "familial" factors, meaning genetic factors. Even among these patients, a causative gene can only be identified in about 50%. A gene called "optineurin" that our research group found in Japanese patients in 2010 is one of the major causative genes of ALS discovered to date. Optineurin was previously known as the gene responsible for the eye disease glaucoma. When functioning normally, optineurin suppresses intracellular nuclear factor kappa B (NF-κB) signaling, but we found that it loses this ability when mutated. NF-κB is known to be involved in inflammation and carcinogenesis in the human body. We found that such loss of optineurin function may have affected the motor nerves in our patients with ALS by causing overactivation of NF-κB. Other researchers later found that optineurin is involved in autophagy as well, which has further increased its importance. A paper our research team published in Nature has been cited more than 800 times, which shows how researchers around the world are using our findings as the basis for a variety of studies.
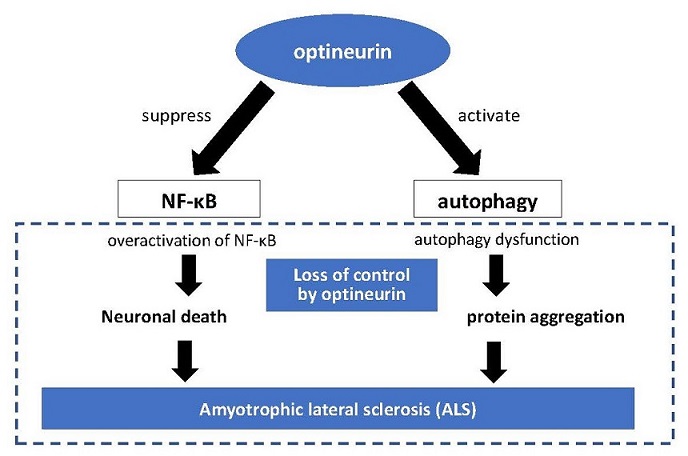
Involvement of optineurin in neurological diseases
Pursuing further discoveries using a third-generation sequencer
We are currently conducting research to determine the mechanism by which the optineurin gene causes ALS. We have genetically engineered mice with the mutation we found in ALS patients, and are continuing to observe these mice to determine whether the mutation will cause ALS in the same way, and if so, what symptoms the mice will exhibit. Neurodegenerative diseases do not develop until a certain age. Even mice, which have a lifespan of about 2 years, do not develop ALS immediately, so we will need to monitor the mice for their entire lifespan.
When we were conducting our research to find optineurin around 2005, it was not long after the successful sequencing of the entire human genome. Therefore, it took a great deal of time and effort to discover a causative gene. However, the situation has changed dramatically over the past decade or so with the rapid advancement of gene-reading devices called “next-generation sequencers.” Ten years ago, it took 10 days and about 5 million yen to analyze all the genes of one person. Today, it only takes about 1 day and 100,000 yen. This has led to the discovery of a series of new causative genes for ALS. In addition, today's "third-generation" sequencers are far better at reading long genome sequences than their predecessors. As studies have shown that neurological diseases such as ALS develop when mutations involving 200 or 300 repeats of a certain base occur, the introduction of third-generation sequencers is expected to help researchers better understand the pathogenic mechanisms of these diseases. Our research group just started using a third-generation sequencer about two years ago as well.

Previously unknown "dark" regions of the genome are being illuminated thanks to the development of third-generation sequencers.
Conducting cutting-edge research in competition with researchers from around the world
The ultimate goal of our research is to find a breakthrough treatment for ALS, for which no "silver bullet" currently exists. NF-κB was not thought to be related to ALS until we discovered optineurin, but now that this relationship is apparent, drugs that inhibit NF-κB are becoming candidates for ALS treatment. We would like to continue our research in a similar way, creating paths to development of treatments based on pathogenic mechanisms.
Basic medical research like ours always involves fierce competition with rivals around the world. In order to get ahead of researchers from other countries and make important discoveries, I tell my students to work on the most important problems of the time, while always taking in the latest information. Rapid advances continue to be made in medical science and biology, and with those advances come more research projects to undertake and more problems to be solved. I would like to continue working with my colleagues in the laboratory to tackle such difficult but rewarding challenges.

 Home
Home