Reference article: Kofuji, S., Hirayama, A., Sasaki, A., et al. IMP dehydrogenase-2 drives aberrant nucleolar activity and promotes tumorigenesis in glioblastoma, Nature Cell Biology, volume 21, pages 1003–1014(2019) DOI: doi.org/10.1038/s41556-019-0363-9
Original text by University of Cincinnati. Edited by Emma Buchet, Hiroshima University.
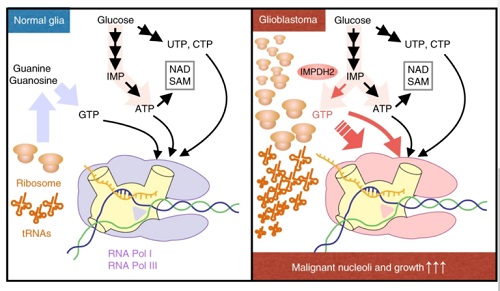
From the paper IMPDH2 upregulation is critical for nucleolar transformation in GBM. Nature Cell Biology
UC researchers unlock cancer cells’ feeding mechanism, central to tumor growth
The findings could lead to new treatments by blocking tumor growth at its roots.
An international team led by researchers from the University of Cincinnati and Japan’s Keio and Hiroshima universities has discovered the energy production mechanism of cancerous cells that drives the growth of the nucleolus and causes tumors to rapidly multiply.
The findings, published Aug. 1 in the journal Nature Cell Biology, could lead to the development of new cancer treatments that would stop tumor growth by cutting the energy supply to the nucleolus.
“The nucleolus is the ‘eye’ of the cancer storm that ravages patients’ bodies. Being able to control the eye would be a true game-changer in cancer treatment,” said Atsuo Sasaki, PhD, associate professor at the UC College of Medicine, visiting professor at Hiroshima University and one of the research team’s lead investigators.
The nucleolus, located near the center of the nucleus, produces ribosomes. The discovery that cancerous cells have enlarged nucleoli occurred over 100 years ago, and studies have since shown that nucleolus enlargement results in significant ribosome increases, propelling protein synthesis to mass produce cancer cells, according to Sasaki. But, exactly how the nucleolus produces a massive amount of ribosome in cancerous cells has largely remained a mystery, he says.
“Nucleolus enlargement is a telltale sign of cancer, and its size has long been used as a yardstick to determine how advanced cancer is in patients,” says Sasaki. “Now, our research team knows that the nucleolus quickly expands by devouring Guanosine Triphosphate, or GTP, a nucleotide and one of the building blocks needed to create RNA, which is prevalent in cancerous cells.”
“We were surprised to find out that among all types of energy that could be used for cell growth, it’s GTP that spikes and plays the most crucial role in ribosome increases that are associated with nucleolus enlargement in cancer cells. We knew right away that this was a substantial discovery that would require a sweeping range of expertise to understand what it truly meant,” Sasaki adds.
Sasaki says researchers saw an elevated level of inosine monophosphate dehydrogenase 2 (IMPDH2) in cancer cells, which accelerates GTP production and in turn fuels nucleolus growth. This is a major step toward solving the mystery surrounding nucleolus growth in cancer cells, he says.
To conduct the research, the multidisciplinary team zeroed in on the energy production pathways in malignant brain tumors and glioblastoma, the deadliest type of brain cancer, in animal models, followed by cohort studies for human specimens. The results showed a significant increase of GTP, which is a form of energy, in glioblastoma. Experts took a deeper look at brain tumor cells and determined that the significantly elevated level of IMPDH2 in cancer cells accelerates GTP production.
The close relationship between IMPDH2 and the nucleolus was discovered and prompted the research team to develop a new method of metabolic analysis, which enabled researchers to obtain critical data for demonstrating that GTP produced from IMPDH activities is used for the nucleolus’s ribosome synthesis; this led to the discovery of a clear correlation between the suppression of glioblastoma cell growth and IMPDH inhibition, which prolonged animal models’ lives.
“Thanks to our multinational cross-disciplinary collaboration and the team’s hard work, we were able to unlock the mechanism through which cancerous cells hijack GTP metabolism to take control over the nucleoli. We are excited to continue our research on GTP for the development of therapies to annihilate the ‘eye of cancer’ in patients,” Sasaki says.
Co-collaborators on the study include Satoshi Kofuji, PhD, Naoya Sakamoto, MD, PhD, and Wataru Yasui, MD, PhD, all of Hiroshima University; Tomoyoshi Soga, PhD, Hideyuki Saya, MD, PhD, and Makoto Suematsu, MD, PhD, all of Keio University; Ralph DeBerardinis, MD, PhD, and Robert Bachoo, MD, PhD, both of UT Southwestern; Ichiro Nakano, MD, PhD, University of Alabama; Hiroaki Wakimoto, MD, PhD, Harvard Medical School; William Young, PhD, UCLA; Craig Horbinski, MD, PhD, Northwestern University; Risa Kawaguchi, PhD, National Institute of Advanced Industrial Science and Technology of Japan; Ingrid Grummt, PhD, the German Cancer Research Center; and Holger Bierhoff, PhD, Friedrich Schiller University.
The work is supported in-part by Home for Innovative Researchers and Academic Knowledge Users (HIRAKU), JSPS KAKENHI grant number JP18K07233 and the Kanae Foundation (SK).
Norifumi Miyokawa
Research Planning Office, Hiroshima University
E-mail: pr-research*office.hiroshima-u.ac.jp (Please change * into @)

 Home
Home



