E-mail: pr-research*office.hiroshima-u.ac.jp (Please replace * with @)
Water is an abundant and essential compound, found everywhere on earth. Yet despite its familiarity and simple structure, water displays many unusual physical properties. For more than a century, scientists have turned their attention to the study of water, attempting to better interpret its structure. An international team of researchers, led by a scholar from Hiroshima University, has developed a procedure allowing them to reproduce the double peak feature of x-ray emission spectroscopy (XES) spectra in liquid water.
The study helping to advance the understanding of the structure of water, led by Osamu Takahashi, an associate professor at Hiroshima University’s Graduate School of Advanced Science and Engineering, is published on February 25 in Physical Review Letters.
Water molecule in liquid phase accelerating dynamics by X-ray radiation (Image courtesy of Osamu Takahashi, Hiroshima University)
Through the years, as scientists have worked to better understand the structure of liquid water, some have studied water using a two-structure model. Other scientists, in a wide range of fields, have used a uniform, continuous liquid model. XES has proven to be a useful tool for researchers studying substances whose features are not homogeneous.
For over a decade, scientists have debated how to interpret XES spectra of liquid water. To solve this problem the research team performed molecular dynamics calculations to create the model structures of liquid water. Their next step was to estimate XES spectra for the liquid water, using first principles of quantum mechanical calculations.
The team was able to theoretically reproduce the double 1b1 feature, present in liquid water’s x-ray emission spectroscopy. They explored different effects, such as geometry and dynamics, to determine the shape of the XES spectra.
Adopting classical molecular dynamics simulations, the team was able to construct the water’s structure in the liquid phase. In these simulations, the researchers worked at various temperature points with the bond length and water molecule angles fixed. In the spectra they calculated, the researchers were able to reproduce the features, such as the double peaks of the 1b1 state, that had been previously observed by other scientists in experimental XES spectra.
To better understand the features they were seeing, the research team classified the XES spectra they calculated based on the different types of hydrogen bonds. They observed the double peak feature in the XES spectra in all the different types of hydrogen bonds they studied.
After examining the spectra related to the hydrogen bonds, the team studied the effect of thermally excited vibrational modes on the XES spectra. They obtained nine independent vibrational modes and studied their effects on the spectra.
The researchers were able to successfully reproduce the XES spectra of liquid water by examining the effect of full vibrational modes, O-H stretching, bending, and rotational modes. They explained both the temperature and isotope dependence by examining the hydrogen-bond configuration around the excited water molecule and core-hole induced dynamics. “Our procedure is general and can be applicable for various systems related to the phenomena including liquid water,” Takahashi said.
The team is hopeful that their research may help to resolve some of the long-standing debates surrounding the interpretation of liquid water’s structure. Looking to the future, the researchers see various potential applications for their procedure. “Development of new materials such as electrodes used in batteries, biomaterials such as artificial blood vessels, and functional polymers such as water treatment membranes may be fascinating projects, which are related to the structure of liquid water,” Takahashi said.
The research team led by Osamu Takahashi, included Ryosuke Yamamura from the Department of Chemistry, Hiroshima University, Japan; Takashi Tokushima from MAX IV Laboratory, Lund University, Sweden; and Yoshihisa Harada from the Institute for Solid State Physics and Synchrotron Radiation Research Organization, University of Tokyo, Japan. The Japanese Society for the Promotion of Science funded this research.
Additional Multimedia:
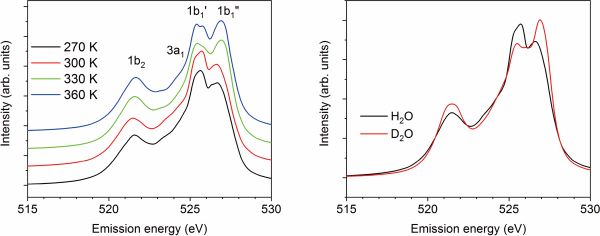
(left) Theoretical XES spectra of water at different temperatures. Two 1b1 states are assigned as 1b1’ and 1b1”. Intensities of 1b1” are increasing with temperature, whereas those of 1b1’ are decreasing, which is the same trend with the experiment. (right) Isotope dependence of theoretical XES spectra of liquid water at 300 K. The same isotope effect can be seen in the experiment, with 1b1’ peak having much lower intensity than the 1b1” for deuterium water D2O, whereas for H2O situation is reversed.
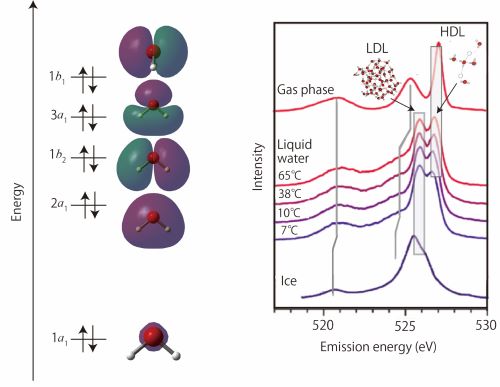
(left) Molecular orbitals of water. (right) XES spectra with various temperature. Two prominent peaks shown in Figure 1 are indicated as LDL and HDL, respectively (this figure is partly modified in the paper written by Tokushima et al., Chem. Phys. Lett., 460, 387 (2008)).
About the study
Journal: Physical Review Letters
Title: Interpretation of the x-ray emission spectra of liquid water through temperature and isotope dependence
Authors: Osamu Takahashi, Ryosuke Yamamura, Takashi Tokushima & Yoshihisa Harada
DOI: 10.1103/PhysRevLett.128.086002
Norifumi Miyokawa
Office of Research and Academia-Government-Community Collaboration, Hiroshima University


 Home
Home