E-mail: pr-research*office.hiroshima-u.ac.jp (Please replace * with @)
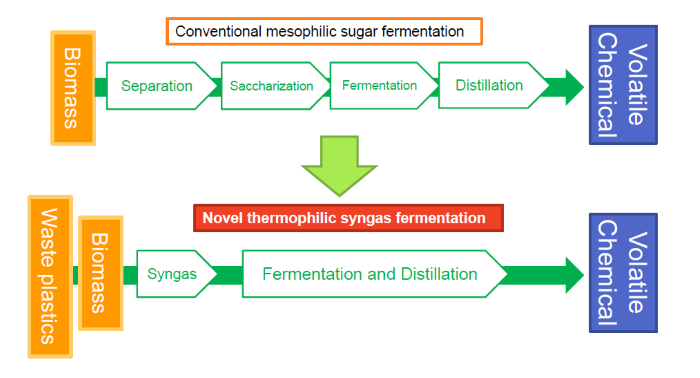
Scientists from Hiroshima University and AIST in Japan engineered the bacterium Moorella thermoacetica to produce a volatile chemical from gaseous substrates at high temperature. It will realize economical thermophilic syngas fermentation process to produce bulk chemical from organic matters and wastes.
Acetone, a volatile solvent used for everything from removing nail polish and cleaning textiles to manufacturing plastics, could get a sustainability boost from a new strain of bacteria engineered by a research team based in Japan.
They published the details of the heat-loving, acetone-producing bacteria called Moorella thermoacetica on April 23 in AMB Express.
Acetone is typically produced through the widely used cumene method, which is cost-effective but not sustainable. The process, developed in 1942, involves converting two non-renewable resources into acetone and phenol, another chemical that helps manufacture a number of materials, including plastics.
More environmentally friendly options exist — including gas fermentation, a bioprocess that converts carbon dioxide, monoxide and hydrogen into chemicals and fuels — but they tend to be cumbersome and costly, according to Yutaka Nakashimada, professor in the Graduate School of Integrated Sciences for Life, Hiroshima University, who led the research. One of the major expenses is the downstream processing, which involves separating out the desired chemicals from the other materials.
“We thought the key is a simultaneous separation of the product from the ongoing fermentation,” Nakashimada said. “Our choice was to produce volatile chemicals by using a group of bacteria thriving at high temperatures.”
The bacteria, M. thermoacetica, eat the gaseous feedstocks of hydrogen, carbon dioxide and monoxide — which can be procured from renewable resources — to produce acetone. Since they grow at a temperature higher than the boiling point of acetone, the acetone produced is a gas that evaporates and can be distilled as the bacteria make it. It streamlines the traditional system into a simultaneous process.
“Our development of the engineered bacteria could pave the way for developing a consolidated process with simplified and cost-effective recovery via condensation following gas fermentation on a large scale suitable for industrial production,” said paper co-first author Junya Kato, specially appointed assistant professor in the Graduate School of Integrated Sciences for Life, Hiroshima University.
To develop this productive bacteria strain, the researchers genetically engineered bacteria with modified metabolism processes.
“To our knowledge, this is the first study to provide strains of bacteria that thrive at high temperatures for gas fermentation of acetone,” Kato said. “Although further study would be needed to improve the productivity for realization of the industrial applications, the gas fermentation process can be simpler and more cost-effective than before.”
The researchers plan to scale their work and study the productivity of their bacteria in industrial conditions.
“We may need to genetically engineer the metabolism of the strain further,” Nakashimada said. “Our ultimate goal is the industrialization of the gas fermentation of the ‘gas-to-gas’ process that is simpler and lower-cost.”
Co-authors include co-first author Kaisei Takemura, Setsu Kato, and Yoshiteru Aoi, the Graduate School of Integrated Sciences for Life at Hiroshima University; and Tatsuya Fujii, Keisuke Wada, Yuki Iwasaki, Akinori Matsushika and Katsuji Murakami, the National Institute of Advanced Industrial Science and Technology (AIST). Matsushika is also affiliated with the Graduate School of Integrated Sciences for Life at Hiroshima University.
The Japan Science and Technology Agency supported this research.
About the study
Journal: AMB Express
Title: Metabolic engineering of Moorella thermoacetica for thermophilic bioconversion of gaseous substrates to a volatile chemical
Authors: Junya Kato, Kaisei Takemura, Setsu Kato, Tatsuya Fujii, Keisuke Wada, Yuki Iwasaki, Yoshiteru Aoi, Akinori Matsushika, Katsuji Murakami & Yutaka Nakashimada
DOI: 10.1186/s13568-021-01220-w
- Profile of Professor Yutaka Nakashimada
- Profile of Assistant Professor (Special Appointment) Junya Kato
Norifumi Miyokawa
Office of Research and Academia-Government-Community Collaboration, Hiroshima University

 Home
Home