Email: bprc*hiroshima-u.ac.jp (Please replace * with @)
Date & Time: October. 19, 2023
Program
Commentary: Yukihiko MATSUMURA
Professor, Graduate School of Advanced Science and Engineering, Hiroshima University

Lecture: Ken FURUTA
B4, School of Engineering, Hiroshima University
“Highly dispersed zinc oxide catalyst for biodiesel synthesis under supercritical methanol conditions”
Biodiesel is attracting attention as an alternative fuel to diesel oil because it is sustainable and carbon neutral. However, conventional biodiesel synthesis methods have problems with long reaction times and large amounts of wastewater. Therefore, the use of catalysts under supercritical methanol conditions was designed to solve these problems. In this study, carbon nanotube-supported zinc oxide catalysts were used under supercritical methanol conditions, and a significant improvement in biodiesel yield was achieved.

Lecture: Mizuki KODAMA
B4, School of Engineering, Hiroshima University
“Effect of catalyst amount on carbon gasification efficiency of supercritical water gasification of glucose”
Supercritical water gasification (SCWG) is a promising technology for efficiently converting biomass into useful gases. In this process, catalysts play a crucial role in accelerating the reaction and improving gas yields. To enhance the efficiency and cost-effectiveness of SCWG, researchers have turned to carbon nanotubes (CNTs) and other carbon-based materials as catalyst supports. In this study, the effect of catalyst amount on supercritical water gasification of glucose was studied experimentally. Carbon gasification efficiency was calculated, and compared to the model prediction. Acknowledgements: This work was supported by JSPS KAKENHI Grant Number JP22K18313.

Lecture: Mohammed Ahmed Mohammed ALI
D3, Graduate School of Advanced Science and Engineering, Hiroshima University
“Reaction rate determination of glucose gasification in supercritical water using CNT and Ru/CNT catalysts”
Carbon nanotubes (CNT), with their unique structure, recently became a good candidate to be used in the catalyst field. On the other hand, a ruthenium (Ru)-based catalyst with CNT support (Ru/CNT) showed great performance in the catalytic gasification of glucose. The reaction rates from the gasification of glucose should be determined for both CNT and Ru/CNT catalysts. However, reaction rate analysis using space time that is often used for catalytic reaction can be changed by catalyst mass and/or feedstock flow rate. This study clarified the difference between these two ways of changing space time, and it is recommended only catalyst mass or only feedstock flow rate is changed.

Lecture: Shunsuke KOMATSU
M1, Graduate School of Integrated Sciences for Life, Hiroshima University
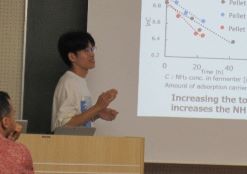
“Development of Methane Fermentation from Organic Wastes with Ammonia Recovery Process”
In this study, a methane fermentation process with ammonia recovery using ammonia adsorbent was investigated. To investigate the effect of hydrothermal treatment on methane productivity from livestock wastes, methanogenic potential tests were conducted on hydrothermally treated chicken manure, beef cattle manure, dairy cattle manure, and swine manure. The results showed that hydrothermally treated swine manure was superior for methane fermentation. Therefore, hydrothermally treated swine manure was subjected to methane fermentation in a 10 L reactor. Ammonia recovery tests using the fermentation residue demonstrated that zirconium phosphate pellets were effective as an ammonia adsorbent.
Chair: Yukihiko MATSUMURA Professor, Graduate School of Advanced Science and Engineering, Hiroshima University
HOSTY Association (Graduate School of Advanced Science and Engineering)

 Home
Home



