Key points of this research results
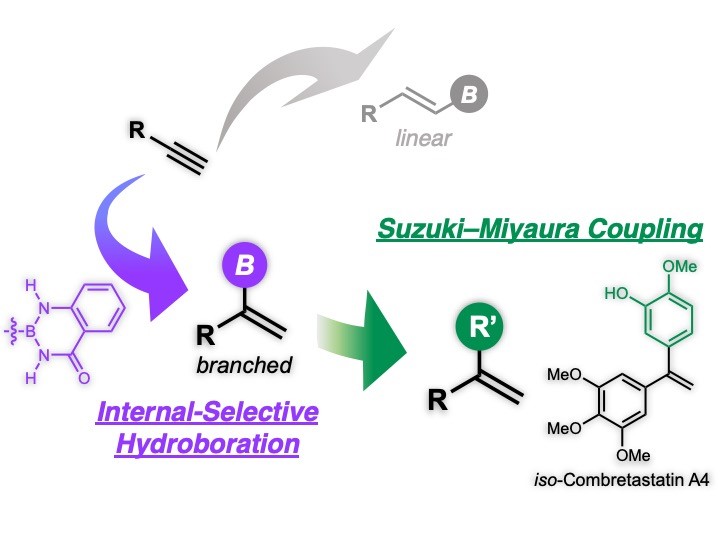
- Installation of a Suzuki–Miyaura coupling(SMC)-active boron functionality into an internal carbon of terminal alkynes was achieved.
- “Diminishment of boron-Lewis acidity” and “ligand-derived steric bulk around a copper center” have proven to be the key to the internal-selectivity.
- Shortcut total synthesis of biologically active iso-Combretastatin A4 was accomplished.
Outline
SMC (the Nobel Prize in Chemistry in 2010) of organoboron compounds has now become one of the most fundamental methods of constructing carbon–carbon bonds that form molecular skeletons of organic compounds. Hence, organoboron compounds are indispensable synthetic intermediates for producing organic functional materials, agrochemicals, pharmaceuticals, etc.
One of the most typical synthetic routes to organoboron compounds is hydroboration of terminal alkynes, whose regioselectivity is governed by anti-Markovnikov selectivity arising from the boron-Lewis acidity. Therefore, we can easily synthesize linear alkenylboron compounds through the boron-installation into the terminal carbon of the triple bonds. Because the inherent boron-Lewis acidity-derived anti-Markovnikov selectivity is a universal rule that is covered even in elementary organic chemistry classes, reversal of this regioselectivity via the boron-installation into the internal carbon has been a challenging issue in modern synthetic organic chemistry.
The results of this research would provide a promising clue to new borylation reactions and shortcut synthesis of various useful molecules.
We have succeeded in installing a boron functionality, being utilizable for SMC, into the internal carbon of the terminal alkynes; two origins governing the internal regioselectivity of the hydroboration have been revealed.
<Origins of internal regioselectivity in copper-catalyzed borylation of terminal alkynes>
① Diminishment of boron-Lewis acidity
② Ligand-derived steric bulk around a copper center
The present Markovnikov-selective hydroboration combined with the direct SMC enabled two-step total synthesis of biologically active iso-Combretastatin A4 that previously required four-steps.

 Home
Home



