Interview of Associate Professor Naoki TARUTANI
Researchers selected for JST FOREST in the FY2022

Combining Nanoparticles like Building Blocks to Create Novel Functional Materials
Assembling Nanoparticles Will Lead to Discovery of Novel Functions
My area of expertise is inorganic material chemistry. The materials we use in our daily lives can be divided into three main categories: metallic, organic, and inorganic. Metallic materials are primarily composed of metallic elements, while organic materials such as plastics are composed mainly of hydrogen, carbon, and oxygen. All other materials, such as ceramics, are inorganic.
When studying materials engineering at university, I chose to focus on inorganic materials because I was fascinated by the fact that a little of surface modification of a material can add various functions, such as repelling water or reflecting specific kinds of light.
You may have noticed that the 2023 Nobel Prize in Chemistry was won by those doing research into quantum dots, a type of inorganic nanomaterial. Quantum dots are particles about 10 nanometers (one 100,000th of a millimeter) in size in which quantum confinement effect is observed. Humankind has been harnessing the power of quantum dots for millennia without knowing it. For example, the vivid reds seen in stained glass are mostly due to gold quantum dots, and the black dyes used by ancient Romans were created by lead sulfide quantum dots. These days, they are used in a wide variety of applications, including high-definition LEDs, bio-imaging, and solar cells.
It was that potential for development of nanomaterials from inorganic materials that appealed to me as I pursued my research.
Currently, my main focus is on synthesizing inorganic materials for use in energy applications like batteries as well as catalysts for purifying car exhaust gas. In particular, we are working on the development of materials with specific structures in the nanometer to micrometer size range, which is larger than the atomic level.
Among the many possible approaches to nanomaterial development, my research is defined by its focus on nanomaterial structure. Even the most common combination of elements can exhibit novel and very useful functions when the structure is changed, and I study the various patterns of those combinations. It is comparable to Lego: where lots of other nanomaterial researchers look into the shapes of the block itself, I attempt to create novel and useful structures by combining the blocks.
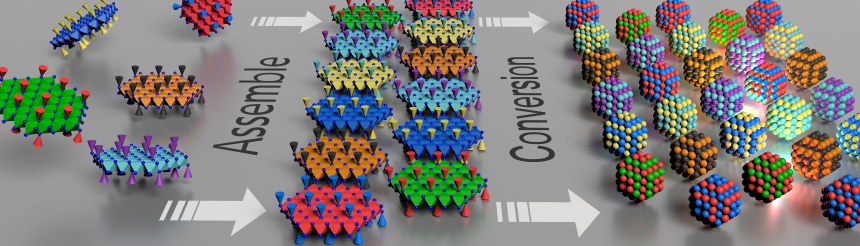
Prof. Tarutani is exploring the potential for discovering novel and useful functions by changing the structure of nanomaterials like Lego blocks.
Endeavoring to Create New Materials That Contribute to Energy and the Environment
Having my project selected for fully seven years of support under the Japan Science and Technology Agency’s Fusion Oriented Research for Disruptive Science and Technology program in 2022 was a tremendous boon for which I am grateful. Hiroshima University has been generous in its support, and I have been assigned a flex-use space laboratory, where I am working on my research with staff and students.

Prof. Tarutani in the laboratory.
Already the project has led to the discovery that when nanoparticles of two inorganic materials, nickel hydroxide and cobalt hydroxide, are synthesized at a size of two nanometers to form a structure, the structure exhibits functions that cannot be observed when synthesis is done at > sub-10 nanometer-scale. These two hydroxides are used as the cathode of nickel-metal hydride batteries and electrochemical catalysts. Mixing nickel and cobalt at the atomic level is known to improve battery performance; we found that mixing them at the micrometer scale (one 1000th of a millimeter) does not result in such an improvement but that mixing them at the nanoparticle level improves battery performance just as much as at the atomic level. We hypothesize that the structure created by the accumulating nanoparticles caused this phenomenon, and are currently conducting analysis.
Moving forward, I hope to create materials that can replace precious metals such as platinum in applications such as decomposing exhaust gases and producing hydrogen through water electrolysis. In this age of global warming, all eyes are on hydrogen as an alternative energy source to fossil fuels, and developing replacements for expensive precious metals will be a significant contribution to the environment.
Chasing the Potential of Complex Composite Clusters
This project entails three main tasks: synthesis of nanomaterials and assemblies, analysis of their structures, and development of their functions. At present, we are developing synthesis methods, and are in the process of creating sub-10-nm nanoparticles of non-precious metal materials such as iron, nickel, and aluminum, as well as inorganic materials such as sulfides and nitrides. We believe that we may be able to create new functions by combining these nanoparticles as building blocks in the future.
The “complex composite clusters” of the project title is a neologism that refers to the way various nanoparticles (i.e., complex) are combined (clustered) in certain order and dimensionalities (composite). The project’s aim is to come up with complex composite clusters that can produce outcomes in the next few years leading to the development of new, world-changing materials.


 Home
Home

